Chemical stability
Considering the chemical properties of gasoline, it is necessary to focus on how long the composition of hydrocarbons will remain unchanged, since with long storage, lighter components disappear and performance is greatly reduced.
In particular, the problem is acute if a higher grade fuel (AI 95) was obtained from gasoline with a minimum octane number by adding propane or methane to its composition. Their antiknock qualities are higher than that of isooctane, but they also dissipate instantly.
According to GOST, the chemical composition of fuel of any brand must be unchanged for 5 years, subject to storage rules. But in fact, often even the newly purchased fuel already has an octane number below the specified one.
Unscrupulous sellers are to blame for this, who add liquefied gas to containers with fuel, the storage time of which has expired, and the content does not meet the requirements of GOST. Usually, different amounts of gas are added to the same fuel to obtain an octane number of 92 or 95. Confirmation of such tricks is the pungent smell of gas at the filling station.
Flash point determination methods
There is a method of open and closed crucible (container for oil products). The temperatures obtained differ due to the amount of accumulated vapors.
The open crucible method includes:
- Cleaning gasoline from moisture using sodium chloride.
- Filling the crucible to a certain level.
- Heating the container to a temperature 10 degrees below the expected result.
- Ignition of a gas burner above the surface.
- At the moment of ignition, the flash point is recorded.
The closed crucible method differs in that the gasoline in the container is constantly mixed. When the lid is opened, the fire is brought up automatically.
The flash point apparatus consists of the following components:
- electric heater (power from 600 watts);
- capacity of 70 milliliters;
- copper stirrer;
- electric or gas igniter;
- thermometer.
Depending on the results, flammable substances are classified:
- especially dangerous (at a flash point below -200C);
- dangerous (from -200C to + 230C);
- dangerous at elevated temperatures (from 230C to 610C).
Speed - Combustion - Fuel
What is the real cost of 1 liter of gasoline
The fuel combustion rate increases greatly if the combustible mixture is in intense vortex (turbulent) motion. Accordingly, the intensity of turbulent heat transfer can be much higher than that of molecular diffusion.
The combustion rate of fuel depends on a number of reasons discussed later in this chapter and, in particular, on the quality of mixing of fuel with air. The rate of fuel combustion is determined by the amount of fuel burned per unit of time.
The combustion rate of the fuel and, therefore, the heat release rate are determined by the size of the combustion surface. Coal dust with a maximum particle size of 300 - 500 microns has a combustion surface tens of thousands of times larger than coarse sorted chain grate fuel.
The rate of fuel combustion depends on the temperature and pressure in the combustion chamber, increasing with their increase. Therefore, after ignition, the combustion rate increases and becomes very high at the end of the combustion chamber.
The speed of fuel combustion is also influenced by the engine speed. With an increase in the number of revolutions, the duration of the phase is reduced.
The turbulence of the gas flow sharply increases the rate of fuel combustion due to an increase in the area of the combustion surface and the speed of propagation of the flame front with an increase in the rate of heat transfer.
When running on a lean mixture, the combustion rate is slowed down. Therefore, the amount of heat given off by gases to parts increases and the engine overheats. Signs of a lean mixture are flashes in the carburetor and intake manifold.
The turbulence of the gas flow sharply increases the rate of fuel combustion due to an increase in the combustion surface area and the speed of propagation of the flame front due to an increase in the rate of heat transfer.
Normal alkanes have the maximum cetane number, which characterizes the rate of fuel combustion in an engine.
The composition of the working mixture greatly affects the rate of combustion of fuel in the engine. These conditions take place at coeff.
The influence of the quality of the development of the combustion process is determined by the rate of fuel combustion in the main phase. When a large amount of fuel is burned in this phase, the values of pz and Tz increase, the proportion of after-burning fuel decreases during the expansion process, and the polytrope index nz becomes larger. This development of the process is most favorable, since the best heat utilization is achieved.
In the working process of the engine, the value of the rate of fuel combustion is very important. The combustion rate is understood as the amount (mass) of fuel reacting (burning) per unit of time.
A number of general phenomena indicate that the rate of fuel combustion in engines is quite natural, not random. This is indicated by the reproducibility of more or less unambiguous cycles in the engine cylinder, which, in fact, determines the stable operation of the engines. In the same engines, the protracted nature of combustion is always observed with lean mixtures. Hard work of the engine, which occurs at a high rate of combustion reactions, is observed, as a rule, in compressorless diesel engines, and soft work - in engines with ignition from an electric spark. This indicates that fundamentally different mixture formation and ignition cause a regular change in the combustion rate. With an increase in the engine speed, the duration of combustion decreases in time, and in the angle of rotation of the crankshaft, it increases. The kinetic curves of the course of burnup in engines are similar in nature to the kinetic curves of a number of chemical reactions that are not directly related to engines and occurring under different conditions.
Experiments indicate the dependence of the intensity of radiant heat transfer on the rate of fuel combustion. With rapid combustion at the root of the torch, higher temperatures develop and heat transfer intensifies. The inhomogeneity of the temperature field, along with different concentrations of emitting particles, leads to inhomogeneity of the degree of flame blackness. All of the above creates great difficulties for the analytical determination of the temperature of the radiator and the degree of emissivity of the furnace.
With a laminar flame (see Section 3 for more details), the fuel combustion rate is constant and Q 0; the combustion process is silent. However, if the combustion zone is turbulent, and this is the case under consideration, then even if the fuel consumption is constant on average, the local combustion rate changes in time and for a small volume element Q.Q. Turbulence is continually disturbing the flame; at any given moment, the combustion is limited by this flame or a series of flames occupying a random position in the combustion zone.
Gaseous fuel
Gaseous fuel is a mixture of various gases: methane, ethylene and other hydrocarbons, carbon monoxide, carbon dioxide or carbon dioxide, nitrogen, hydrogen, hydrogen sulfide, oxygen and other gases, as well as water vapor.
Methane (CH4) is the main constituent of many natural gases. Its content in natural gases reaches 93 ... 98%. Combustion of 1 m3 of methane releases ~ 35 800 kJ of heat.
Gaseous fuels can also contain small amounts of ethylene (C2H4). Combustion of 1 m3 of ethylene gives ~ 59,000 kJ of heat.
In addition to methane and ethylene, gaseous fuels also contain hydrocarbon compounds, such as propane (C3H8), butane (C4H10), etc. The combustion of these hydrocarbons produces more heat than the combustion of ethylene, but their amount is insignificant in combustible gases.
Hydrogen (H2) is 14.5 times lighter than air. The combustion of 1 m3 of hydrogen releases ~ 10 800 kJ of heat. Many combustible gases, other than coke oven gas, contain relatively small amounts of hydrogen. In coke oven gas, its content can reach 50 ... 60%.
Carbon monoxide (CO) is the main combustible component of blast furnace gas. The combustion of 1 m3 of this gas produces ~ 12,770 kJ of heat. This gas is colorless, odorless and highly toxic.
Hydrogen sulfide (H2S) is a heavy gas with an unpleasant odor and is highly toxic. In the presence of hydrogen sulfide in the gas, the corrosion of the metal parts of the furnace and the gas pipeline increases. The harmful effect of hydrogen sulfide is enhanced by the presence of oxygen and moisture in the gas. Combustion of 1 m3 of hydrogen sulfide releases ~ 23 400 kJ of heat.
The rest of the gases: CO2, N2, O2 and water vapor are ballast components, since with an increase in the content of these gases in the fuel, the content of its combustible components decreases. Their presence leads to a decrease in the combustion temperature of the fuel. A content of> 0.5% free oxygen in gaseous fuels is considered hazardous for safety reasons.
Boiling - gasoline
Octane number Gasoline composition
Gasoline starts boiling at a relatively low temperature and proceeds very intensively.
The end of the boiling point of gasoline is not specified.
The beginning of boiling of gasoline is below 40 C, the end is 180 C, the temperature of the beginning of crystallization is not higher than 60 C. The acidity of gasoline does not exceed 1 mg / 100 ml.
The end boiling point of gasoline according to GOST is 185 C, and the actual one is 180 C.
The end-boiling point of gasoline is the temperature at which a standard (100 ml) portion of the test gasoline is completely distilled (boiled away) from the glass flask in which it was located into the refrigerator-receiver.
| Stabilization installation diagram. |
The final boiling point of gasoline should not exceed 200 - 225 C. For aviation gasolines, the final boiling point is much lower, reaching in some cases up to 120 C.
MPa, the boiling point of gasoline is 338 K, its average molar mass is 120 kg / kmol, and the heat of vaporization is 252 kJ / kg.
The initial boiling point of gasoline, for example 40 for aviation gasoline, indicates the presence of light, low-boiling fractions, but does not indicate their content. The boiling point of the first 10% fraction, or starting temperature, characterizes the starting properties of gasoline, its volatility, as well as the tendency to form gas locks in the gasoline supply system. The lower the boiling point of the 10% fraction, the easier it is to start the engine, but also the greater the possibility of the formation of gas locks, which can cause interruptions in the fuel supply and even stop the engine. Too high boiling point of the starting fraction makes it difficult to start the engine at low ambient temperatures, which leads to losses of gasoline.
| Influence of the end point of boiling point of gasoline on its consumption during vehicle operation. The effect of the distillation temperature of 90% gasoline on the octane number of gasolines of various origins. |
A decrease in the end of the boiling point of reforming gasolines leads to a deterioration in their detonation resistance. Research and economic calculations are needed to address this issue.It should be noted that in the foreign practice of a number of countries, motor gasolines with a boiling point of 215 - 220 C are currently being produced and used.
| Influence of the end point of boiling point of gasoline on its consumption during vehicle operation. Influence of the distillation temperature of 90% gasoline on the octane number of gasolines of various origins. |
A decrease in the end of the boiling point of reforming gasolines leads to a deterioration in their detonation resistance. Research and economic calculations are needed to address this issue. It should be noted that in the foreign practice of a number of countries, motor gasolines with a boiling point of 215 - 220 C are currently being produced and used.
If the end-boiling point of gasoline is high, then the heavy fractions contained in it may not evaporate, and, therefore, not burn out in the engine, which will lead to increased fuel consumption.
Lowering the end-boiling point of straight-run gasolines leads to an increase in their detonation resistance. Low-octane straight-run gasolines have octane numbers of 75 and 68, respectively, and are used as components of motor gasolines.
Combustion - gasoline
Design and principle of operation Bosch Motronic MED 7 direct petrol injection system
Combustion of gasoline, kerosene and other liquid hydrocarbons occurs in the gas phase. Combustion can occur only when the concentration of fuel vapor in the air is within certain limits, individual for each substance. If a small amount of fuel vapors is contained in the IB air, combustion will not occur, as well as in the case when there is too much fuel vapors and not enough oxygen.
| Temperature change on the surface of kerosene during extinguishing with foams. | Temperature distribution in kerosene before the start of extinguishing (a and at the end. |
When gasoline burns, as is known, a homothermal layer is formed, the thickness of which increases with time.
When gasoline burns, water and carbon dioxide are formed. Can this serve as sufficient confirmation that gasoline is not an element?
When gasoline, kerosene and other liquids are burned in tanks, the crushing of the gas flow into separate volumes and the combustion of each of them separately are especially clearly visible.
When gasoline and oil are burned in large-diameter tanks, the character of heating differs significantly from that described above. When they burn, a heated layer appears, the thickness of which naturally increases over time and the temperature is the same as the temperature on the surface of the liquid. Under it, the temperature of the liquid drops rapidly and becomes almost the same as the initial temperature. The nature of the curves shows that during combustion, gasoline breaks down into two layers - an upper and a lower one.
For example, burning gasoline in air is called a chemical process. In this case, energy is released, equal to approximately 1300 kcal per 1 mole of gasoline.
Analysis of the combustion products of gasoline and oils is becoming extremely important, since knowledge of the individual composition of such products is necessary for the study of combustion processes in the engine and for the study of air pollution.
Thus, when gasoline is burned in wide tanks, up to 40% of the heat released as a result of combustion is consumed for radiation.
Table 76 shows the burning rate of gasoline with tetranitro-methane additives.
Experiments have shown that the speed of gasoline burning from the surface of the tank is significantly influenced by its diameter.
| Alignment of forces and means when extinguishing a fire on the stretch. |
With the help of GPS-600, firefighters successfully coped with the elimination of the burning of gasoline that spilled along the railway track, ensuring the movement of the trunk operators to the place where the tanks were coupled.Having disconnected them, with a piece of a contact wire, they attached 2 tanks with gasoline to the fire engine and pulled them out of the fire zone.
| The rate of heating of oils in tanks of various diameters. |
A particularly large increase in the speed of warming up from the wind was noticed when burning gasoline. When gasoline was burning in a tank of 2 64 m at a wind speed of 1 3 m / s, the heating rate was 9 63 mm / min, and at a wind speed of 10 m / s, the heating rate increased to 17 1 mm / min.
Ignition temperature and other parameters
The combustion of coal is a chemical reaction of carbon oxidation that occurs at a high initial temperature with intense heat release. Now it is simpler: coal fuel cannot ignite like paper; preheating to 370-700 ° C is required for ignition, depending on the brand of fuel.
Key moment. The efficiency of coal combustion in a furnace or a household solid fuel boiler is characterized not by the maximum temperature, but by the completeness of combustion. Each carbon molecule combines with two oxygen particles in the air to form carbon dioxide CO2. The process is reflected in the chemical formula.
If you limit the amount of incoming oxygen (cover the blower, switch the TT-boiler to smoldering mode), instead of CO2, carbon monoxide CO is formed and emitted into the chimney, the combustion efficiency will significantly decrease. To achieve high efficiency, it is necessary to provide favorable conditions:
- Brown coals ignite at a temperature of +370 ° C, stone - 470 ° C, anthracite - 700 degrees. Pre-heating of the heating unit with wood (sawdust briquettes) is required.
- Air is supplied to the firebox in excess, the safety factor is 1.3-1.5.
- Combustion is supported by the high temperature of a hot bed of coals lying on the grate. It is important to ensure the passage of oxygen through the entire thickness of the fuel, since air moves through the ash pan due to the natural chimney draft.
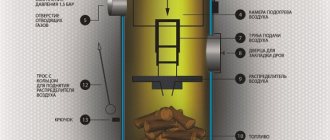
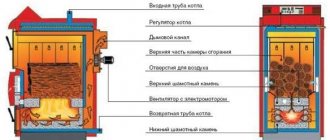
Comment. The only exceptions are home-made Bubafonya-type stoves and cylindrical boilers for upper combustion, where air is fed into the firebox from top to bottom.
The theoretical combustion temperature and specific heat transfer of various fuels are shown in the comparative table. It is noticeable that, under ideal conditions, any fuel will release maximum heat when interacting with the required volume of air.
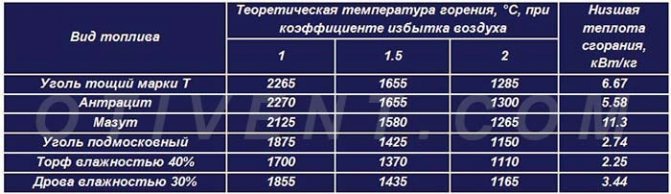

In practice, it is unrealistic to create such conditions, so the air is supplied with some excess. The real combustion temperature of brown coal in a conventional TT-boiler is within 700 ... 800 ° C, stone and anthracite - 800 ... 1100 degrees.
If you overdo it with the amount of oxygen, the energy will begin to be spent on heating the air and simply fly out into the pipe, the efficiency of the furnace will noticeably drop. Moreover, the temperature of the fire can reach 1500 ° C. The process resembles an ordinary fire - the flame is large, there is little heat. An example of efficient combustion of coal with a retort burner on an automatic boiler is presented in the video:
Temperature - combustion - fuel
| Dependence of criterion B on the ratio of the area of heat sources to the area of the workshop. |
The intensity of the worker's irradiation depends on the combustion temperature of the fuel in the furnace, the size of the charging hole, the thickness of the furnace walls at the charging hole and, finally, on the distance at which the worker is from the charging hole.
| The CO / CO and H2 / HO ratios in the products of incomplete combustion of natural gas, depending on the air consumption coefficient a. |
The practically achievable temperature 1L is the combustion temperature of the fuel in real conditions. When determining its value, heat losses to the environment, the duration of the combustion process, the method of combustion and other factors are taken into account.
Excess air dramatically affects the combustion temperature of the fuel.So, for example, the actual combustion temperature of natural gas with a 10% excess of air is 1868 C, with a 20% excess of 1749 C and with a 100% excess of air, it decreases to 1167 C. On the other hand, the preheating of air, going to the combustion of fuel, increases the temperature of its combustion. So, when burning natural gas (1Max 2003 C) with air heated to 200 C, the combustion temperature rises to 2128 C, and when the air is heated to 400 C - up to 2257 C.
| General diagram of the furnace device. |
When air and gaseous fuel are heated, the combustion temperature of the fuel rises, and, consequently, the temperature of the working space of the furnace also rises. In many cases, reaching the temperatures required for a given technological process is impossible without high heating of air and gaseous fuel. For example, steel smelting in open-hearth furnaces, for which the temperature of the torch (flow of burning gases) in the melting space should be 1800 - 2000 C, would be impossible without heating air and gas to 1000 - 1200 C. When heating industrial furnaces low-calorie local fuel (damp firewood, peat, brown coal), their work without heating the air is often even impossible.
It can be seen from this formula that the combustion temperature of the fuel can be increased by increasing its numerator and decreasing the denominator. The dependence of the combustion temperature of various gases on the excess air ratio is shown in Fig.
Excess air also sharply affects the combustion temperature of the fuel. So, the heat output of natural gas with an excess of air of 10% - 1868 C, with an excess of air of 20% - 1749 C and with a 100% excess is equal to 1167 C.
If the hot junction temperature is limited only by the combustion temperature of the fuel, the use of recuperation makes it possible to increase the temperature Тт by increasing the temperature of the combustion products and thus increase the overall efficiency of the TEG.
The enrichment of the blast with oxygen leads to a significant increase in the combustion temperature of the fuel. As the graph data in Fig. 17, the theoretical temperature of fuel combustion is associated with the enrichment of the blast with oxygen by a dependence, which is practically linear up to the oxygen content in the blast of 40%. At higher degrees of enrichment, the dissociation of combustion products begins to have a significant effect, as a result of which the curves of the temperature dependence on the degree of enrichment of the blast deviate from straight lines and asymptotically approach the temperatures limiting for a given fuel. Thus, the considered dependence of the fuel combustion temperature on the degree of oxygen enrichment of the blast has two regions - a region of relatively low enrichments, where there is a linear dependence, and a region of high enrichments (over 40%), where the temperature rise has a decaying character.
An important thermotechnical indicator of the furnace operation is the furnace temperature, which depends on the combustion temperature of the fuel and the nature of heat consumption.
The ash of the fuel, depending on the composition of the mineral impurities, at the temperature of the combustion of the fuel can be fused into pieces of slag. The characteristic of fuel ash depending on temperature is given in table. BUT.
The value of tmaK in table. IV - З - calorimetric (theoretical) temperature of fuel combustion.
Heat losses through the walls of the furnaces to the outside (into the environment) reduce the combustion temperature of the fuel.
Combustion of fuel
Fuel combustion is a process of oxidation of combustible components that occurs at high temperatures and is accompanied by the release of heat. The nature of combustion is determined by many factors, including the method of combustion, the design of the furnace, the concentration of oxygen, etc. But the conditions of the course, the duration and the final results of combustion processes largely depend on the composition, physical and chemical characteristics of the fuel.
Fuel composition
Solid fuels include coal and brown coal, peat, oil shale, wood. These types of fuels are complex organic compounds formed mainly by five elements - carbon C, hydrogen H, oxygen O, sulfur S and nitrogen N. The fuel also contains moisture and non-combustible minerals, which form ash after combustion. Moisture and ash are the external ballast of the fuel, while oxygen and nitrogen are internal.
The main element of the combustible part is carbon, it determines the release of the greatest amount of heat. However, the greater the proportion of carbon in a solid fuel, the more difficult it is to ignite. During combustion, hydrogen releases 4.4 times more heat than carbon, but its share in the composition of solid fuels is small. Oxygen, not being a heat-generating element and binding hydrogen and carbon, reduces the heat of combustion, therefore it is an undesirable element. Its content is especially high in peat and wood. The amount of nitrogen in solid fuels is small, but it is capable of forming oxides that are harmful to the environment and humans. Sulfur is also a harmful impurity, it emits little heat, but the resulting oxides lead to corrosion of the metal of the boilers and pollution of the atmosphere.
Fuel specifications and their influence on the combustion process
The most important technical characteristics of fuel are: heat of combustion, yield of volatile substances, properties of non-volatile residue (coke), ash content and moisture content.
Heat of combustion of fuel
The calorific value is the amount of heat released during the complete combustion of a unit of mass (kJ / kg) or volume of fuel (kJ / m3). Distinguish between higher and lower heat of combustion. The highest includes the heat released during the condensation of vapors contained in the combustion products. When fuel is burned in boiler furnaces, the exhaust flue gases have a temperature at which moisture is in a vaporous state. Therefore, in this case, a lower heat of combustion is used, which does not take into account the heat of condensation of water vapor.
The composition and net calorific value of all known coal deposits have been determined and given in the calculated characteristics.
Volatile matter release
When solid fuel is heated without access to air under the influence of high temperature, water vapor is first released, and then thermal decomposition of molecules occurs with the release of gaseous substances, called volatile substances.
The release of volatile substances can occur in the temperature range from 160 to 1100 ° C, but on average - in the temperature range of 400-800 ° C. The temperature of the beginning of the release of volatiles, the amount and composition of gaseous products depend on the chemical composition of the fuel. The chemically older the fuel is, the lower the release of volatiles and the higher the temperature of their onset of release.
Volatiles provide earlier ignition of the particulate matter and have a significant effect on fuel combustion. Fuels young in age - peat, brown coal - ignite easily, burn quickly and almost completely. Conversely, fuels with low volatiles, such as anthracite, are more difficult to ignite, burn much more slowly and do not burn completely (with increased heat loss).
Non-volatile residue (coke) properties
The solid part of the fuel remaining after the release of volatiles, consisting mainly of carbon and a mineral part, is called coke. The coke residue can be, depending on the properties of organic compounds included in the combustible mass: caked, weakly caked (destroyed by exposure), powdery. Anthracite, peat, brown coal give a powdery non-volatile residue. Most bituminous coals are sintered, but not always strongly. Sticky or powdery non-volatile residue gives bituminous coals with a very high yield of volatiles (42-45%) and with a very low yield (less than 17%).
The structure of the coke residue is important when burning coal in grate furnaces.When flaring in power boilers, the coke performance is not very important.
Ash content
Solid fuel contains the largest amount of non-combustible mineral impurities. These are primarily clay, silicates, iron pyrite, but iron oxide, sulfates, carbonates and silicates of iron, oxides of various metals, chlorides, alkalis, etc. can also be included. Most of them fall during mining in the form of rocks, between which coal seams lie, but there are also mineral substances that have passed into the fuel from coal-formers or in the process of converting its original mass.
When fuel is burned, mineral impurities undergo a series of reactions, as a result of which a solid non-combustible residue called ash is formed. The weight and composition of the ash are not identical to the weight and composition of the mineral impurities of the fuel.
Ash properties play an important role in the organization of boiler and furnace operation. Its particles, carried away by the combustion products, at high speeds abrade the heating surfaces, and at low speeds they are deposited on them, which leads to a deterioration in heat transfer. Ash carried away into the chimney can harm the environment; to avoid this, the installation of ash collectors is required.
An important property of ash is its fusibility; they distinguish between refractory (above 1425 ° C), medium-melting (1200-1425 ° C) and low-melting (less than 1200 ° C) ash. Ash that has passed the stage of melting and turned into a sintered or fused mass is called slag. The temperature characteristic of the ash fusibility is of great importance for ensuring the reliable operation of the furnace and boiler surfaces; the correct choice of the temperature of the gases near these surfaces will eliminate slagging.
Moisture content
Moisture is an undesirable component of the fuel; it, along with mineral impurities, is ballast and reduces the content of the combustible part. In addition, it reduces the thermal value, since additional energy is required for its evaporation.
The moisture in the fuel can be internal or external. External moisture is contained in the capillaries or trapped on the surface. With chemical age, the amount of capillary moisture decreases. The smaller the pieces of fuel, the greater the surface moisture. Internal moisture enters the organic matter.
The moisture content in the fuel reduces the heat of combustion and leads to an increase in fuel consumption. At the same time, the volumes of combustion products increase, heat losses with exhaust gases increase and the efficiency of the boiler unit decreases. High humidity in winter leads to freezing of coal, difficulties in grinding and a decrease in flowability.
Fuel combustion methods depending on the type of furnace
The main types of combustion devices:
- layered,
- chamber.
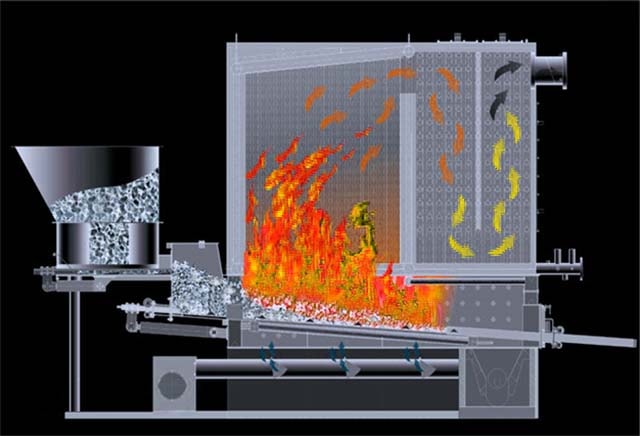
Layer furnaces are intended for combustion of lumpy solid fuel. They can be dense and fluidized. When burning in a dense layer, the combustion air passes through the layer without affecting its stability, that is, the gravity of the burning particles exceeds the dynamic pressure of the air. When burned in a fluidized bed, due to the increased air velocity, the particles go into a "boiling" state. In this case, active mixing of the oxidizer and the fuel occurs, due to which the combustion of the fuel is intensified.
AT chamber furnaces burn solid pulverized fuel, as well as liquid and gaseous. Chamber furnaces are subdivided into cyclonic and flare ones. During flare combustion, coal particles should be no more than 100 microns, they burn in the volume of the combustion chamber. Cyclonic combustion allows a larger particle size; under the influence of centrifugal forces, they are thrown onto the walls of the furnace and completely burn out in a swirling flow in a high temperature zone.
Combustion of fuel. The main stages of the process
In the process of burning solid fuel, certain stages can be distinguished: heating and evaporation of moisture, sublimation of volatiles and the formation of coke residue, combustion of volatiles and coke, and the formation of slag. This division of the combustion process is relatively arbitrary, since although these stages proceed sequentially, they partially overlap each other. So, the sublimation of volatile substances begins before the final evaporation of all moisture, the formation of volatiles occurs simultaneously with the process of their combustion, just as the onset of oxidation of the coke residue precedes the end of the combustion of volatiles, and the afterburning of coke can also proceed after the formation of slag.
The flow time of each stage of the combustion process is largely determined by the properties of the fuel. The coke combustion stage lasts the longest, even for fuels with a high volatile yield. Various operating factors and design features of the furnace have a significant impact on the duration of the stages of the combustion process.
1. Preparation of fuel before ignition
The fuel entering the furnace is heated, as a result of which, in the presence of moisture, it evaporates and the fuel dries up. The time required for heating and drying depends on the amount of moisture and the temperature at which the fuel is supplied to the combustion device. For fuels with a high moisture content (peat, wet brown coals), the heating and drying stage is relatively long.
Fuel is supplied to stacked furnaces at a temperature close to the ambient temperature. Only in winter, when coal freezes, its temperature is lower than in the boiler room. For combustion in flare and vortex furnaces, the fuel is subjected to crushing and grinding, accompanied by drying with hot air or flue gases. The higher the temperature of the incoming fuel, the less time and heat is needed to heat it up to the ignition temperature.
Fuel drying in the furnace occurs due to two heat sources: convective heat of combustion products and radiant heat of a torch, lining, and slag.
In chamber furnaces, heating is carried out mainly due to the first source, that is, admixing combustion products to the fuel at the point of its entry. Therefore, one of the important requirements for the design of devices for introducing fuel into the furnace is to ensure intensive suction of combustion products. A higher temperature in the firebox also contributes to a shorter heating and drying time. To this end, when burning fuels with the beginning of the release of volatiles at high temperatures (more than 400 ° C), incendiary belts are made in chamber furnaces, that is, they close the shield pipes with a refractory heat-insulating material in order to reduce their heat perception.
When burning fuel in a bed, the role of each type of heat source is determined by the design of the furnace. In furnaces with chain grates, heating and drying are carried out mainly by the radiant heat of the torch. In furnaces with a fixed grate and fuel supply from above, heating and drying occurs due to combustion products moving through the layer from the bottom up.
In the process of heating at a temperature above 110 ° C, thermal decomposition of organic substances that make up the fuels begins. The least strong compounds are those that contain a significant amount of oxygen. These compounds decompose at relatively low temperatures with the formation of volatiles and a solid residue, consisting mainly of carbon.
Fuels that are young in chemical composition, containing a lot of oxygen, have a low temperature of the beginning of the release of gaseous substances and give a higher percentage of them. Fuels with a low content of oxygen compounds have a low volatility yield and a higher flash point.
The content of molecules in solid fuels, which readily decompose when heated, also affects the reactivity of the non-volatile residue.First, the decomposition of the combustible mass occurs mainly on the outer surface of the fuel. With further heating, pyrogenetic reactions begin to occur inside the fuel particles, the pressure rises in them and the outer shell breaks. When fuels with a high yield of volatiles are burned, the coke residue becomes porous and has a larger surface compared to the dense solid residue.
2. The combustion process of gaseous compounds and coke
The actual combustion of fuel begins with the ignition of volatile substances. During the fuel preparation period, branched chain reactions of oxidation of gaseous substances occur, at first these reactions proceed at low rates. The released heat is perceived by the surfaces of the furnace and is partially accumulated in the form of energy of moving molecules. The latter leads to an increase in the rate of chain reactions. At a certain temperature, oxidation reactions proceed at such a rate that the heat released completely covers the heat absorption. This temperature is the flash point.
The ignition temperature is not constant, it depends both on the properties of the fuel and on the conditions in the ignition zone, on average it is 400-600 ° C. After ignition of the gaseous mixture, further self-acceleration of oxidation reactions causes an increase in temperature. To maintain combustion, a continuous supply of oxidant and combustible substances is required.
The ignition of gaseous substances leads to the enveloping of the coke particle in a fire envelope. Combustion of coke begins when the combustion of volatiles comes to an end. The solid particle heats up to a high temperature, and as the amount of volatiles decreases, the thickness of the boundary burning layer decreases, oxygen reaches the hot carbon surface.
Combustion of coke begins at a temperature of 1000 ° C and is the longest process. The reason is that, firstly, the oxygen concentration decreases, and secondly, heterogeneous reactions proceed more slowly than homogeneous ones. As a result, the duration of combustion of a solid fuel particle is determined mainly by the combustion time of the coke residue (about 2/3 of the total time). For fuels with a high volatility yield, the solid residue is less than ½ of the initial particle mass, therefore, their combustion occurs quickly and the possibility of underburning is low. Chemically old fuels have a dense particle, the combustion of which takes almost the entire time spent in the furnace.
The coke residue of most solid fuels is mainly, and for some species, entirely composed of carbon. The combustion of solid carbon occurs with the formation of carbon monoxide and carbon dioxide.
Optimal conditions for heat dissipation
The creation of optimal conditions for the combustion of carbon is the basis for the correct construction of a technological method for burning solid fuels in boiler units. The following factors can influence the achievement of the highest heat release in the furnace: temperature, excess air, primary and secondary mixture formation.
Temperature... Heat release during fuel combustion depends significantly on the temperature regime of the furnace. At relatively low temperatures, incomplete combustion of combustible substances takes place in the torch core; carbon monoxide, hydrogen, and hydrocarbons remain in the combustion products. At temperatures from 1000 to 1800-2000 ° C, complete combustion of the fuel is achievable.
Excess air... Specific heat generation reaches its maximum value with complete combustion and an excess air ratio of unity. With a decrease in the excess air ratio, the heat release decreases, since the lack of oxygen leads to the oxidation of less fuel. The temperature level decreases, the reaction rates decrease, which leads to a sharp decrease in heat release.
An increase in the excess air ratio greater than unity reduces heat generation even more than a lack of air.In real conditions of fuel combustion in boiler furnaces, the limiting values of heat release are not reached, since there is incomplete combustion. It largely depends on how the mixture formation processes are organized.
Mixing processes... In chamber furnaces, primary mixing is achieved by drying and mixing fuel with air, supplying part of the air (primary) to the preparation zone, creating a wide-open torch with a wide surface and high turbulization, using heated air.
In layered furnaces, the primary mixing task is to supply the required amount of air to different combustion zones on the grate.
In order to ensure the afterburning of gaseous products of incomplete combustion and coke, processes of secondary mixture formation are organized. These processes are facilitated by: the supply of secondary air at a high speed, the creation of such aerodynamics, in which uniform filling of the entire furnace with a torch is achieved and, consequently, the residence time of gases and coke particles in the furnace increases.
3. Slag formation
In the process of oxidation of the combustible mass of solid fuel, significant changes also occur in mineral impurities. Low-melting substances and alloys with a low melting point dissolve refractory compounds.
A prerequisite for the normal operation of boilers is the uninterrupted removal of combustion products and the resulting slag.
During layer combustion, slag formation can lead to mechanical underburning - mineral impurities envelop unburned coke particles, or viscous slag can block air passages, blocking oxygen access to the burning coke. To reduce underburning, various measures are used - in furnaces with chain grates, the time spent on the slag grate is increased, and frequent shuraing is performed.
In layered furnaces, the slag is removed in dry form. In chamber furnaces, slag removal can be dry or liquid.
Thus, fuel combustion is a complex physicochemical process, which is influenced by a large number of different factors, but all of them must be taken into account when designing boilers and furnaces.
Combustion - gasoline
Combustion of gasoline with detonation is accompanied by the appearance of sharp metal knocks, black smoke on the exhaust, an increase in gasoline consumption, a decrease in engine power and other negative phenomena.
The combustion of gasoline in the engine also depends on the excess air ratio. At the values a 0 9 - j - 1 1, the rate of pre-flame oxidation processes in the working mixture is the highest. Therefore, at these values of a, the most favorable conditions are created for the onset of detonation.
After the combustion of gasoline, the total mass of such pollutants increased significantly along with the general redistribution of their quantities. The percentage of benzene in the condensate of automobile exhaust gases was about 1 to 7 times higher than that in gasoline; the toluene content was 3 times higher, and the xylene content 30 times higher. It is known that oxygen compounds are formed in this case, and the number of ions, characteristic of heavier unsaturated compounds of the olefin or cycloparaffin series and acetylene or diene series, especially the latter, increases sharply. Generally speaking, the changes to the Haagen-Smit chamber resembled the changes needed to make the composition of typical vehicle exhaust samples similar to those of the Los Angeles smog sample.
The calorific value of gasoline depends on its chemical composition. Therefore, hydrocarbons rich in hydrogen (for example, paraffinic ones) have a large mass heat of combustion.
Gasoline combustion products expand in the internal combustion engine along the polytrope n1 27 from 30 to 3 at. The initial temperature of gases is 2100 C; the mass composition of combustion products of 1 kg of gasoline is as follows: CO23 135 kg, H2 1 305 kg, O20 34 kg, N2 12 61 kg.Determine the work of expansion of these gases, if 2 g of gasoline is fed into the cylinder at the same time.
| Influence of TPP on carbon formation in the engine. |
When gasoline is burned from a thermal power plant, carbon deposits are formed containing lead oxide.
When gasoline is burned in reciprocating internal combustion engines, almost all of the resulting products are carried away with the exhaust gases. Only a relatively small part of the products of incomplete combustion of fuel and oil, a small amount of inorganic compounds formed from elements introduced with fuel, air and oil, are deposited in the form of carbon deposits.
When gasoline is burned with tetraethyl lead, lead oxide is apparently formed, which melts only at a temperature of 900 C and can evaporate at a very high temperature, exceeding the average temperature in the engine cylinder. To prevent the deposition of lead oxide in the engine, special substances are introduced into the ethyl fluid - scavengers. The halogenated hydrocarbons are used as scavengers. Usually these are compounds containing bromine and chlorine, which also burn and bind lead in new bromide and chloride compounds.
| Influence of TPP on carbon formation in the engine. |
When gasoline is burned from a thermal power plant, carbon deposits are formed containing lead oxide.
During the combustion of gasoline containing pure TPP, a plaque of lead compounds is deposited in the engine. The composition of ethyl liquid grade R-9 (by weight): tetraethyl lead 54 0%, bromoethane 33 0%, monochloronaphthalene 6 8 0 5%, filler - aviation gasoline - up to 100%; dye dark red 1 g per 1 kg of the mixture.
When gasoline containing TPP is burned, fistula oxide with low volatility is formed in the engine; since the melting point of lead oxide is quite high (888), part of it (about 10%, counting on lead introduced with gasoline) is deposited as a solid residue on the walls of the combustion chamber, candles and valves, which leads to a rapid engine failure.
When gasoline is burned in a car engine, smaller molecules are also formed and the released energy is distributed in a larger volume.
Gases incandescent from the combustion of gasoline flow around the heat exchanger 8 (inside from the side of the combustion chamber and further, through the windows 5 outside, passing through the exhaust gas chamber 6) and heat the air in the heat exchanger channel. Next, hot exhaust gases are fed through the exhaust pipe 7 under the sump and heat up the engine from the outside, and hot air from the heat exchanger is fed through the breather into the crankcase and heats up the engine from the inside. In 1 5 - 2 minutes after the start of heating, the glow plug is switched off and combustion in the heater continues without its participation. After 7 - 13 minutes from the moment of receiving a pulse to start the engine, the oil in the crankcase warms up to a temperature of 30 C (at an ambient temperature of up to -25 C) and the unit start-up pulses are supplied, after which the heater turns off.
Combustion temperature
In heat engineering, the following combustion temperatures of gases are distinguished: heat output, calorimetric, theoretical and actual (calculated). Heating capacity tx is the maximum temperature of the products of complete gas combustion in adiabatic conditions with an excess air coefficient a = 1.0 and at a gas and air temperature equal to 0 ° C:
tx = Qh / (IVcv) (8.11)
where QH is the lowest calorific value of gas, kJ / m3; IVcp - the sum of the products of the volumes of carbon dioxide, water vapor and nitrogen formed during the combustion of 1 m3 of gas (m3 / m3) and their average volumetric heat capacities at constant pressure within the temperature range from 0 ° С to tx (kJ / (m3 * ° С ).
Due to the inconstancy of the heat capacity of gases, the heat output is determined by the method of successive approximations. As the initial parameter, its value for natural gas (= 2000 ° C) is taken, with a = 1.0, the volumes of the components of combustion products are determined, according to table.8.3, their average heat capacity is found and then, according to the formula (8.11), the heat capacity of the gas is calculated. If, as a result of the calculation, it turns out to be lower or higher than the accepted one, then a different temperature is set and the calculation is repeated. The heat output of common simple and complex gases when they burn in dry air is given in table. 8.5. When burning gas in atmospheric air containing about 1 wt. % moisture, heat production decreases by 25-30 ° С.
The calorimetric combustion temperature tK is the temperature determined without taking into account the dissociation of water vapor and carbon dioxide, but taking into account the actual initial temperature of the gas and air. It differs from the heat output tx in that the gas and air temperatures, as well as the excess air coefficient a, are taken from their actual values. You can determine tK by the formula:
tк = (Qн + qphys) / (ΣVcp) (8.12)
where qphys is the heat content (physical heat) of gas and air, measured from 0 ° C, kJ / m3.
Natural and liquefied petroleum gases are usually not heated before combustion, and their volume compared to the volume of combustion air is small.
Table 8.3.
Average volumetric heat capacity of gases, kJ / (m3 • ° С)
| Ttemperature, ° С | CO2 | N2 | O2 | CO | CH4 | H2 | H2O (water vapor) | air | |
| dry | wet per m3 dry gas but | ||||||||
| 0 | 1,5981 | 1,2970 | 1,3087 | 1,3062 | 1,5708 | 1,2852 | 1,4990 | 1,2991 | 1,3230 |
| 100 | 1,7186 | 1,2991 | 1,3209 | 1,3062 | 1,6590 | 1,2978 | 1,5103 | 1,3045 | 1,3285 |
| 200 | 1,8018 | 1,3045 | 1,3398 | 1,3146 | 1,7724 | 1,3020 | 1,5267 | 1,3142 | 1,3360 |
| 300 | 1,8770 | 1,3112 | 1,3608 | 1,3230 | 1,8984 | 1,3062 | 1,5473 | 1,3217 | 1,3465 |
| 400 | 1,9858 | 1,3213 | 1,3822 | 1,3356 | 2,0286 | 1,3104 | 1,5704 | 1,3335 | 1,3587 |
| 500 | 2,0030 | 1,3327 | 1,4024 | 1,3482 | 2,1504 | 1,3104 | 1,5943 | 1,3469 | 1,3787 |
| 600 | 2,0559 | 1,3453 | 1,4217 | 1,3650 | 2,2764 | 1,3146 | 1,6195 | 1,3612 | 1,3873 |
| 700 | 2,1034 | 1,3587 | 1,3549 | 1,3776 | 2,3898 | 1,3188 | 1,6464 | 1,3755 | 1,4020 |
| 800 | 2,1462 | 1,3717 | 1,4549 | 1,3944 | 2,5032 | 1,3230 | 1,6737 | 1,3889 | 1,4158 |
| 900 | 2,1857 | 1,3857 | 1,4692 | 1,4070 | 2,6040 | 1,3314 | 1,7010 | 1,4020 | 1,4293 |
| 1000 | 2,2210 | 1,3965 | 1,4822 | 1,4196 | 2,7048 | 1,3356 | 1,7283 | 1,4141 | 1,4419 |
| 1100 | 2,2525 | 1,4087 | 1,4902 | 1,4322 | 2,7930 | 1,3398 | 1,7556 | 1,4263 | 1,4545 |
| 1200 | 2,2819 | 1,4196 | 1,5063 | 1,4448 | 2,8812 | 1,3482 | 1,7825 | 1,4372 | 1,4658 |
| 1300 | 2,3079 | 1,4305 | 1,5154 | 1,4532 | — | 1,3566 | 1,8085 | 1,4482 | 1,4771 |
| 1400 | 2,3323 | 1,4406 | 1,5250 | 1,4658 | — | 1,3650 | 1,8341 | 1,4582 | 1,4876 |
| 1500 | 2,3545 | 1,4503 | 1,5343 | 1,4742 | — | 1,3818 | 1,8585 | 1,4675 | 1,4973 |
| 1600 | 2,3751 | 1,4587 | 1,5427 | — | — | — | 1,8824 | 1,4763 | 1,5065 |
| 1700 | 2,3944 | 1,4671 | 1,5511 | — | — | — | 1,9055 | 1,4843 | 1,5149 |
| 1800 | 2,4125 | 1,4746 | 1,5590 | — | — | — | 1,9278 | 1,4918 | 1,5225 |
| 1900 | 2,4289 | 1,4822 | 1,5666 | — | — | — | 1,9698 | 1,4994 | 1,5305 |
| 2000 | 2,4494 | 1,4889 | 1,5737 | 1,5078 | — | — | 1,9694 | 1,5376 | 1,5376 |
| 2100 | 2,4591 | 1,4952 | 1,5809 | — | — | — | 1,9891 | — | — |
| 2200 | 2,4725 | 1,5011 | 1,5943 | — | — | — | 2,0252 | — | — |
| 2300 | 2,4860 | 1,5070 | 1,5943 | — | — | — | 2,0252 | — | — |
| 2400 | 2,4977 | 1,5166 | 1,6002 | — | — | — | 2,0389 | — | — |
| 2500 | 2,5091 | 1,5175 | 1,6045 | — | — | — | 2,0593 | — | — |
Therefore, when determining the calorimetric temperature, the heat content of gases can be ignored. When burning gases with a low calorific value (generator, blast furnace, etc.), their heat content (especially heated before combustion) has a very significant effect on the calorimetric temperature.
The dependence of the calorimetric temperature of natural gas of average composition in air with a temperature of 0 ° C and a humidity of 1% on the excess air coefficient a is given in Table. 8.5, for LPG when it is burned in dry air - in table. 8.7. Table data. 8.5-8.7 it is possible to be guided with sufficient accuracy when establishing the calorimetric temperature of combustion of other natural gases, which are relatively similar in composition, and hydrocarbon gases of almost any composition. If it is necessary to obtain a high temperature when burning gases with low excess air coefficients, as well as to increase the efficiency of furnaces, in practice, the air is heated, which leads to an increase in the calorimetric temperature (see Table 8.6).
Table 8.4.
Heating capacity of gases in dry air
| Simple gas | Heating capacity, ° С | Complex gas of average composition | Approximate heating capacity, ° С |
| Hydrogen | 2235 | Natural gas fields | 2040 |
| Carbon monoxide | 2370 | Natural oil fields | 2080 |
| Methane | 2043 | Coke | 2120 |
| Ethane | 2097 | High Temperature Shale Distillation | 1980 |
| Propane | 2110 | Steam-oxygen blast under pressure | 2050 |
| Butane | 2118 | Fat coal generator | 1750 |
| Pentane | 2119 | Generator steam-air blast from lean fuels | 1670 |
| Ethylene | 2284 | Liquefied (50% C3H4 + 50% C4H10) | 2115 |
| Acetylene | 2620 | Water | 2210 |
Table 8.5.
Calorimetric and theoretical temperatures of natural gas combustion in air with t = 0 ° С and humidity 1% * depending on the excess air coefficient a
| Excess air ratio a | Calorimetric combustion temperature tк, ° С | Theoretical combustion temperature | Excess air ratio a | Calorimetric combustion temperature tк, ° С |
| 1,0 | 2010 | 1920 | 1,33 | 1620 |
| 1,02 | 1990 | 1900 | 1,36 | 1600 |
| 1,03 | 1970 | 1880 | 1,40 | 1570 |
| 1,05 | 1940 | 1870 | 1,43 | 1540 |
| 1,06 | 1920 | 1860 | 1,46 | 1510 |
| 1,08 | 1900 | 1850 | 1,50 | 1470 |
| 1,10 | 1880 | 1840 | 1,53 | 1440 |
| 1,12 | 1850 | 1820 | 1,57 | 1410 |
| 1,14 | 1820 | 1790 | 1,61 | 1380 |
| 1,16 | 1800 | 1770 | 1,66 | 1350 |
| 1,18 | 1780 | 1760 | 1,71 | 1320 |
| 1,20 | 1760 | 1750 | 1,76 | 1290 |
| 1,22 | 1730 | — | 1,82 | 1260 |
| 1,25 | 1700 | — | 1,87 | 1230 |
| 1,28 | 1670 | — | 1,94 | 1200 |
| 1,30 | 1650 | — | 2,00 | 1170 |
>
The theoretical combustion temperature tT is the maximum temperature determined similarly to the calorimetric temperature tK, but with a correction for endothermic (requiring heat) reactions of dissociation of carbon dioxide and water vapor, proceeding with an increase in volume:
СО2 ‹–› СО + 0.5О2 - 283 mJ / mol (8.13)
Н2О ‹–› Н2 + 0.5О2 - 242 mJ / mol (8.14)
At high temperatures, dissociation can lead to the formation of atomic hydrogen, oxygen, and OH hydroxyl groups. In addition, combustion of gas always produces some amount of nitrogen oxide. All these reactions are endothermic and lead to a decrease in the combustion temperature.
Table 8.6.
Calorimetric temperature of natural gas combustion tу, ° С, depending on the ratio of excess dry air and its temperature (rounded values)
| Excess air ratio a | Dry air temperature, ° С | ||||||||
| 20 | 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | |
| 0,5 | 1380 | 1430 | 1500 | 1545 | 1680 | 1680 | 1740 | 1810 | 1860 |
| 0,6 | 1610 | 1650 | 1715 | 1780 | 1840 | 1900 | 1960 | 2015 | 2150 |
| 0,7 | 1730 | 1780 | 1840 | 1915 | 1970 | 2040 | 2100 | 2200 | 2250 |
| 0,8 | 1880 | 1940 | 2010 | 2060 | 2130 | 2200 | 2260 | 2330 | 2390 |
| 0,9 | 1980 | 2030 | 2090 | 2150 | 2220 | 2290 | 2360 | 2420 | 2500 |
| 1,0 | 2050 | 2120 | 2200 | 2250 | 2320 | 2385 | 2450 | 2510 | 2560 |
| 1,2 | 1810 | 1860 | 1930 | 2000 | 2070 | 2140 | 2200 | 2280 | 2350 |
| 1,4 | 1610 | 1660 | 1740 | 1800 | 2870 | 1950 | 2030 | 2100 | 2160 |
| 1,6 | 1450 | 1510 | 1560 | 1640 | 1730 | 1800 | 1860 | 1950 | 2030 |
| 1,8 | 1320 | 1370 | 1460 | 1520 | 1590 | 1670 | 1740 | 1830 | 1920 |
| 2,0 | 1220 | 1270 | 1360 | 1420 | 1490 | 1570 | 1640 | 1720 | 1820 |
Table 8.7.
Calorimetric combustion temperature tK of commercial propane in dry air with t = 0 ° С depending on the excess air coefficient a
| Excess air ratio a | Calorimetric combustion temperature tH, ° С | Excess air ratio a | Calorimetric combustion temperature tK, ° С |
| 1,0 | 2110 | 1,45 | 1580 |
| 1,02 | 2080 | 1,48 | 1560 |
| 1,04 | 2050 | 1,50 | 1540 |
| 1,05 | 2030 | 1,55 | 1500 |
| 1,07 | 2010 | 1,60 | 1470 |
| 1,10 | 1970 | 1,65 | 1430 |
| 1,12 | 1950 | 1,70 | 1390 |
| 1,15 | 1910 | 1,75 | 1360 |
| 1,20 | 1840 | 1,80 | 1340 |
| 1,25 | 1780 | 1,85 | 1300 |
| 1,27 | 1750 | 1,90 | 1270 |
| 1,30 | 1730 | 1,95 | 1240 |
| 1,35 | 1670 | 2,00 | 1210 |
| 1,40 | 1630 | 2,10 | 1170 |
The theoretical combustion temperature can be determined using the following formula:
tT = (Qн + qphys - qdis) / (ΣVcp) (8.15)
where qduc is the total heat consumption for the dissociation of СО2 and Н2О in combustion products, kJ / m3; IVcp - the sum of the product of the volume and the average heat capacity of combustion products, taking into account dissociation per 1 m3 of gas.
As you can see from the table. 8.8, at temperatures up to 1600 ° C, the degree of dissociation can be disregarded, and the theoretical combustion temperature can be taken equal to the calorimetric temperature. At higher temperatures, the degree of dissociation can significantly reduce the temperature in the workspace. In practice, there is no particular need for this, the theoretical combustion temperature must be determined only for high-temperature furnaces operating on preheated air (for example, open-hearth furnaces). There is no need for this for boiler plants.
The actual (calculated) temperature of the combustion products td is the temperature that is reached under real conditions at the hottest point of the flame. It is lower than the theoretical one and depends on the heat loss to the environment, the degree of heat transfer from the combustion zone by radiation, the length of the combustion process in time, etc. on the temperature in the furnaces with the introduction of experimentally established correction factors into them:
td = t (8.16)
where n - t. n. pyrometric coefficient within:
- for high-quality thermal and heating furnaces with thermal insulation - 0.75-0.85;
- for sealed furnaces without thermal insulation - 0.70-0.75;
- for shielded boiler furnaces - 0.60-0.75.
In practice, it is necessary to know not only the adiabatic combustion temperatures given above, but also the maximum temperatures occurring in the flame. Their approximate values are usually established experimentally by spectrographic methods. The maximum temperatures arising in a free flame at a distance of 5-10 mm from the top of the conical combustion front are given in table. 8.9. An analysis of the data presented shows that the maximum temperatures in the flame are less than the heat output (due to the consumption of heat for the dissociation of H2O and CO2 and the removal of heat from the flame zone).
- home
- Directory
- Combustion characteristics of gases
- Combustion temperature
Combustion - oil product
Combustion of oil products in the embankment of the tank farm is eliminated by the immediate supply of foam.
Combustion of oil products in the embankment of the tank farm is eliminated by immediate supply of foam.
During the combustion of petroleum products, their boiling point (see Table 69) gradually increases due to the ongoing fractional distillation, in connection with which the temperature of the upper layer also rises.
| K Diagram of a fire-fighting water supply system for cooling a burning tank through an irrigation ring .. |
When burning oil in the tank, the upper part of the upper belt of the tank is exposed to the flame. When burning oil at a lower level, the height of the free side of the tank in contact with the flame can be significant. In this mode of combustion, the reservoir may collapse. Water from fire nozzles or from stationary irrigation rings, falling on the outer part of the upper walls of the tank, cools them (Fig.15.1), thus preventing an accident and spreading of oil into the embankment, creating more favorable conditions for the use of air-mechanical foam.
The results of studying the combustion of petroleum products and their mixtures are interesting.
Its temperature during the combustion of oil products is: gasoline 1200 C, tractor kerosene 1100 C, diesel fuel 1100 C, crude oil 1100 C, fuel oil 1000 C. When burning wood in stacks, the temperature of the turbulent flame reaches 1200 - 1300 C.
Particularly large studies in the field of physics of combustion of petroleum products and their extinguishing have been carried out over the past 15 years at the Central Research Institute of Fire Defense (TsNIIPO), the Energy Institute of the USSR Academy of Sciences (ENIN) and a number of other research and educational institutes.
An example of negative catalysis is the suppression of the combustion of petroleum products with the addition of halogenated hydrocarbons.
Water promotes foaming and the formation of emulsions during the combustion of petroleum products with a flash point of 120 C and higher. The emulsion, covering the surface of the liquid, isolates it from the oxygen in the air, and also prevents the escape of vapors from it.
| Combustion rate of liquefied hydrocarbon gases in isothermal tanks. |
Combustion of liquefied hydrocarbon gases in isothermal tanks does not differ from the combustion of petroleum products. The combustion rate in this case can be calculated by formula (13) or determined experimentally. The peculiarity of the combustion of liquefied gases under isothermal conditions is that the temperature of the entire mass of liquid in the tank is equal to the boiling point at atmospheric pressure. For hydrogen, methane, ethane, propane and butane, these temperatures are, respectively, - 252, - 161, - 88, - 42 and 0 5 C.
| Installation diagram of GVPS-2000 generator on the tank. |
Research and practice of extinguishing fires have shown that in order to stop the combustion of an oil product, the foam must completely cover its entire surface with a layer of a certain thickness. All foams with a low expansion rate are ineffective in extinguishing fires of oil products in tanks at the lower level of flooding. Foam, falling from a great height (6 - 8 m) onto the surface of the fuel, is dipped and enveloped in a film of fuel, burns out or quickly collapses. Only foam with a multiplicity of 70 - 150 can be thrown into a burning tank with hinged jets.
| Fire breaks. |